
A modern approach to high‐speed multiplex imaging for neuroscience
The ability to capture fast events in detail is crucial for driving new discoveries in neuroscience. However, as microscopy technology becomes increasingly advanced, weighing up the available options for achieving high temporal resolution within laboratory budgets can be a challenge. This white paper takes a closer look at widefield fluorescence microscopy and explains how recent developments can achieve lightning‐fast multiplex imaging speeds of under 7 μs with affordable equipment.
Find out:
- Which combination of widefield illumination technologies can achieve <7 μs imaging speeds
- How one filter cube is nearly always sufficient for multiplex fluorescence imaging
- What should be considered when choosing optical filters for speed and contrast
Widefield fluorescence microscopy is a popular method of choice for analysing dynamic events in live samples. While confocal laser scanning techniques have the benefit of optical sectioning, image acquisition speeds are limited by the scanning process, and widefield setups therefore have the potential to offer higher temporal resolution. Furthermore, contrast can be enhanced simply by using image processing software tools as deconvolution. Alternatively, some spinning disk confocal systems provide optical sectioning with the speed of widefield fluorescence – and both methods benefit from LED illumination technology.
In addition to capturing biological processes in more detail, high‐speed imaging increases the accuracy of ratiometric fluorescence measurements, for example when performing Fura‐2 calcium imaging. Speed is also a benefit when it comes to combining imaging with optogenetics.
The maximum speed of a widefield fluorescence imaging setup is determined by several components, but the illumination system is often overlooked when deciding how to optimise a setup for high‐speed imaging. This is now changing, and breakthroughs in LED light sources and optical filter components over the last decade have now made it possible to reach speeds below 7 μs with widefield microscopy.
High‐speed LED illumination
Unlike traditional mercury and metal halide lamps for widefield fluorescence microscopy, LED illumination systems are electronically controlled. Avoiding the latency due to physical shuttering which can be as much as 20 ms for each exposure is the key to high‐speed imaging. However, other factors must be considered to fully realise the speed potential of electronic control in image acquisition, and especially when multiplexing fluorophores in one sample.
TTL control of light source: When operating the illumination system through imaging software, latencies can be introduced by USB serial communications and computer operating system overheads, slowing LED on/off speeds by as much as 100 ms. This may also impact system synchronisation when combining imaging with other techniques. Digital control via signals, such as TTL pulses, is how LED illumination systems, such as the CoolLED pE‐800fura, are able to achieve speeds of under 7 μs, but specialised components, such as USB‐controlled TTL trigger boxes, are required. The simplest approach for TTL control is Sequence Runner (available on selected CoolLED Illumination Systems), which uses the TTL output signal available on most scientific cameras or other hardware and cycles though LEDs in a user‐selected sequence for each TTL signal. Analogue control for irradiance is sometimes available, and is beneficial in cases such as adjusting the power of optogenetic stimulation in an otherwise firmly timed experimental protocol.
Optical filters: A common misconception in multiplex fluorescence imaging is that a trade‐off must be made between speed and contrast. Single‐band filter cubes mounted into a motorised filter cube turret offer high contrast but low speed, whereas multi‐band filter sets offer high speeds but low contrast.
Modern LED illumination systems featuring individual channel switching instead allow the best of both worlds when used with Pinkel filter configurations, using a single‐band excitation filter alongside a multi‐band emission filter and multi‐band dichroic mirror. When the single‐band excitation filters are housed in the illumination system itself, in front of each individual LED channel, this allows the filter cube housing the multi‐band filters to remain stationary and removes mechanical latency from the system. The speed is then limited only by LED channel switching speeds, which as previously mentioned can be as fast as under 7 μs.
Instead of filter cube turrets, compact filter cube modules designed for these setups are available, such as Scientifica’s low‐vibration Filter Cube Module+ designed for neuroscience microscopes. This is a perfect complement to LED illumination systems which include inline excitation filter holders, individual channel control and high‐speed TTL triggering. It then only needs to be populated with three filter cubes for different applications or studies, since one Pinkel filter set is sufficient to image several fluorophores, such as the Chroma Quad band 89404 for imaging DAPI, FITC, TRITC and Cy5.
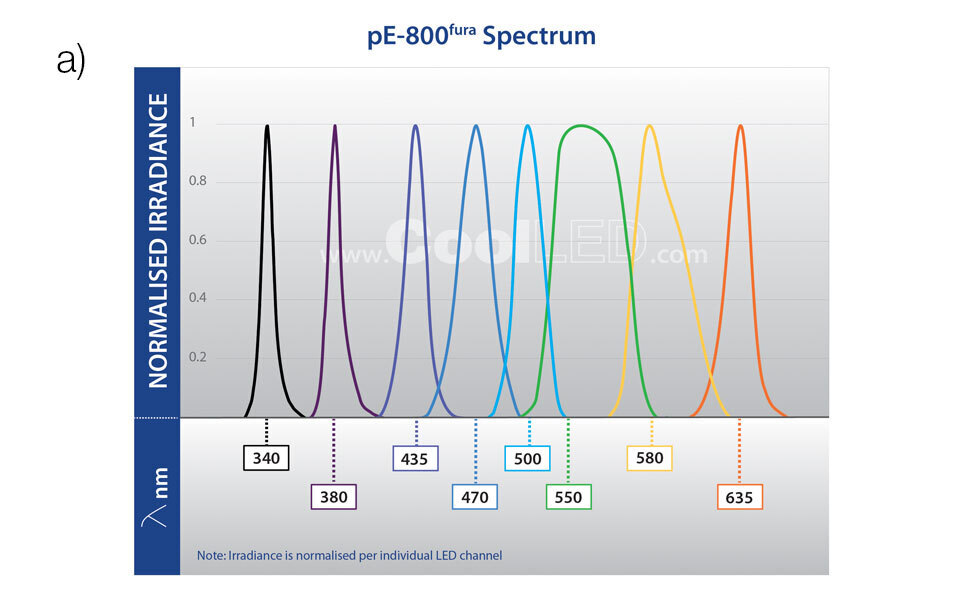
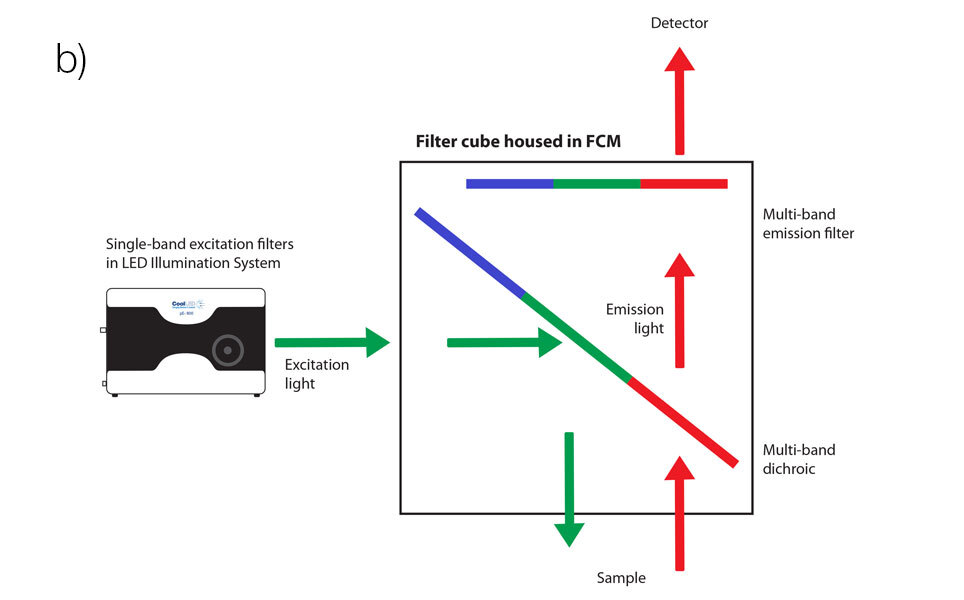
Focusing on filters
Unlike lasers, each LED channel has a broader spectrum which means they offer a much broader coverage of fluorophores. An LED light source is likely to be compatible with new fluorophores becoming available in the future, such as red‐shifted dyes which reduce phototoxicity. However, this also means that the spectrum of LEDs requires careful tailoring to the fluorophore(s) by using optical filters. This is an even greater consideration when using multi‐band filters, which must be chosen carefully to avoid bleed‐through (also known as crosstalk) and a reduction in the signal‐to‐noise ratio. To complicate matters, many filters have been designed around traditional metal halide light sources.
Not everyone has the time to analyse spectral data in order to carefully match optical filters to their illumination system and fluorophore sets, and an easier option is to make use of available online tools such as:
- CoolLED Filter Finder Tool: Find Semrock and Chroma filters optimised for LED illumination systems
- Chroma Spectra Viewer: Find filters and visualise filter and fluorophore spectra
- FPbase: Visualise fluorophores, filters, light sources, detectors
Summary
LED microscopy illumination has disrupted traditional approaches to high‐speed imaging and optogenetic stimulation for neuroscience, and understanding which filters, light sources and other components are right for each individual setup can be confusing.
The aim of this article is to provide a starting point for information, but if you would like to find out more or if you have any questions, please contact [email protected].
References
- Scientifica (2021). Widefield fluorescence microscopy: What you need to know.
- CoolLED (2020). Capturing high‐speed multi‐labelled events with LED illumination systems.
Scientifica Filter Cube Module+
Collect higher quality data across the entire field of view
- Gain higher quality data with more even epifluorescent field illumination
- Improve patch clamp experiments with minimal vibrations
- Compatible with a wide range of light options
CoolLED pE‐800fura
Supercharged calcium imaging and beyond
- Covers all popular calcium indicators, opsins and fluorescent labels
- 8 individually controllable channels
- Broad spectrum from 340 – 635 nm
- <7 μs TTL triggering
- Affordable automation with Sequence Runner
- Software, TTL & analogue control

